For some reason, at least when talking about studies, testosterone right now is hot. And we’re not talking about the usual benefits: muscles, energy, and sex. No, several new studies have recently been released that show how damaging low testosterone levels are to your health–in fact, even to your life expectancy. Note: although the studies mostly focused on the impact of low-T on men’s health, we will also touch on its importance to women’s health as well. And then we’re going to look at a couple of the biggest reasons for having low testosterone–reasons that almost no one thinks about. So, without further ado, let’s look at these studies.
Low testosterone and future mortality risk
Male aging is characterized by a decline in testosterone levels with a substantial variability between subjects. However, it has been unclear whether differences in age-related changes in testosterone are associated with general health and, particularly, mortality rates. A study published in the January edition of the European Journal of Endocrinology attempted to resolve those questions by investigating the associations between mortality and changes in an individual’s serum levels of total testosterone, sex hormone binding globulin, free testosterone, and luteinizing hormone during a ten-year period with up to 18 years of registry follow-up.1 Stine A Holmboe1, Niels E Skakkebæk, Anders Juul, et al. “Individual testosterone decline and future mortality risk in men.” Eur J Endocrinol January 1, 2018 178 121-128. http://www.eje-online.org/content/178/1/121.abstract Note: women experience the same age-related decline in testosterone as men, but since they have so much less to start with, the health implications are even more profound for women than men. And although the study did not look at this issue, we will later in this newsletter.
The study followed 1167 men aged 30–60 years of age for an average of about 15 years (after their original 1993–1994 examination) who were participating in the Danish Monitoring Trends and Determinants of Cardiovascular Disease study. The purpose of the study was to investigate the association between an individual’s hormone changes and all-cause cardiovascular disease and cancer mortalities. Quite simply, the study showed that higher mortality rates were seen among the men who had the most pronounced age-related decline in testosterone, independent of their baseline testosterone levels. Specifically, men whose 10-year decline in testosterone was among the most pronounced experienced a significantly greater risk of dying from any cause over the follow-up period in comparison with most of the other participants. Those whose testosterone declined (on average) more than 1.0 nanomoles per liter per year, which placed them below the 10th percentile, had a 60% greater risk of death during follow-up than those whose change in testosterone placed them between the 10th and 90th percentiles.
“Few other studies have looked at intra-individual declines in testosterone levels in aging men in relation to mortality,” authors Stine A. Holmboe and colleagues noted. “Our findings suggest that a more pronounced age-related decline in testosterone is associated with mortality in men independent of baseline hormone level. A possible causal link between an increased tempo in age-related testosterone decline and subsequent health is unknown and remains to be investigated.”
Higher mortality rates are seen among the men who have the most pronounced age-related decline in testosterone.
Lower testosterone linked to sudden death from heart disease
Lowering testosterone levels to treat cancer, known as androgen deprivation therapy (ADT), is the cornerstone of treatment for about 50 percent of prostate cancer patients. The goal is to reduce levels of the male hormones testosterone and dihydrotestosterone (called androgens) in the body or to stop them from affecting prostate cancer cells. Since androgens stimulate prostate cancer cells to grow, suppressing them with pharmaceutical drugs slows the growth of the cancer. In fact, lowering androgen levels or stopping them from getting into prostate cancer cells often makes prostate cancers shrink or grow more slowly for a time.
It is well known that although ADT results in improved survival in some patients, it has many side effects, including an increased risk of cardiovascular disease and sudden death. The mechanisms by which ADT does this, though, are not well understood. Now, a study recently published in the Journal of the Endocrine Society may have shed light on the issue.2 Thiago Gagliano-Jucá, Thomas G Travison, Philip W Kantoff, et al. “Androgen Deprivation Therapy Is Associated With Prolongation of QTc Interval in Men With Prostate Cancer.” Journal of the Endocrine Society, Volume 2, Issue 5, 1 May 2018, Pages 485–496. http://academic.oup.com/jes/article/2/5/485/4980315
This was a 6-month prospective cohort study that enrolled 33 men with prostate cancer who were about to undergo ADT and a control group of 38 men who had previously had their prostates removed for prostate cancer but had never received ADT.
At study entry, all participants had healthy testicles, which meant they were producing testosterone, and had no history of cardiac arrhythmias and no major problems moving electrical signals through their hearts (AKA a bundle branch block), which would have made it harder for the heart to pump efficiently.
Note: I’m now going to tell you what the study found–then I’ll translate it into English.
ADT was associated with prolongation of the QTc by 7.4 ms compared with the non-ADT group. ADT was also associated with shortening of the QRS interval by 2.4 ms.
Translation: In cardiology, the QTc and QRS are measures of intervals in the human heartbeat. For example, the QT interval represents electrical depolarization and repolarization of the ventricles and is indicated as the time between the Q and T points on the image below. A lengthened QT interval is a marker for the potential of ventricular tachyarrhythmias and a risk factor for sudden death. And as you saw above, the QTc was lengthened by 7.4 ms. You get the idea: men undergoing ADT for prostate cancer experienced prolongation of the QTc. This offers a probable explanation for the increased risk of sudden cardiac death seen in these patients. Or to put it another way, testosterone is known to shorten the time necessary for cells in the heart to be able to contract again after a previous contraction. Reduced testosterone levels because of ADT prolong this time, which is known as the QTc interval on the electrocardiogram. The study found that men receiving ADT experience a prolongation of their QTc interval duration compared with men who do not receive ADT. QTc prolongation is well established to be associated with a higher risk of arrhythmia, suggesting that the prolongation of the QTc interval during ADT might be the mechanism behind some of these cardiac events.
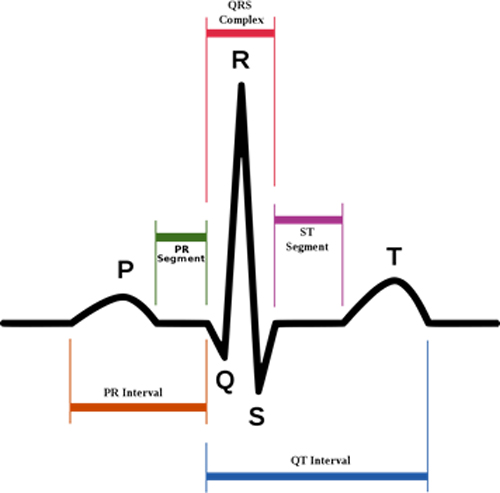
Mechanisms of low testosterone endothelial dysfunction
As we’ve already mentioned, evidence from clinical studies suggests that patients with low testosterone levels are at increased cardiovascular disease risk. Even though the exact mechanisms remain poorly understood, low plasma testosterone is associated with a pro-atherogenic lipid profile, insulin resistance, and increased levels of pro-inflammatory mediators, and vascular dysfunction which is typically observed in patients with hypogonadism (meaning men whose testes produce insufficient testosterone). Furthermore, recent evidence suggests that testosterone deficiency also has direct adverse effects on endothelium and nitric oxide bioavailability. Observations from a study of patients with hypogonadism published in the Hellenic Journal of Cardiology imply that the mechanisms of endothelial dysfunction related to testosterone-deficiency may involve changes in asymmetric dimethylarginine (ADMA) levels. ADMA is a known inhibitor of nitric oxide synthase (proteins in the body that stimulate the production of nitric oxide).3 Antonopoulos AS, Antoniades C. “Mechanisms of testosterone deficiency-related endothelial dysfunction.” Hellenic J Cardiol. 2018 Jun 8. pii: S1109-9666(18)30168-4. http://www.sciencedirect.com/science/article/pii/S1109966618301684?via%3Dihub It is known that men with hypogonadal hypogonadism have higher plasma ADMA levels compared to age-matched men without the condition. Evidence from the study suggests that testosterone replacement therapy is not only a safe, but also an effective, means to reduce atherosclerotic risk and reverse endothelial dysfunction in patients with hypogonadal hypogonadism. Further research in the field is expected to clarify whether changes in ADMA metabolism constitute the central mechanism by which low testosterone leads to endothelial dysfunction. Given the small numbers of patients enrolled in this study (10 men), the findings of Tsikas et al. can be viewed only as preliminary data on the potential role of how low testosterone’s impact on ADMA levels results in the increased cardiovascular risk of patients with hypogonadal hypogonadism.
Testosterone and cardiovascular health, a review
 This was not so much a study as a review of current literature published in the January 2018 issue of the Mayo Clinic Proceedings under the auspices of the Mayo Foundation for Medical Education and Research. According to the review:4 Andrew Elagizi, Tobias S. Köhler, and Carl J. Lavie. “Testosterone and Cardiovascular Health.” Mayo Clin Proc. January 2018;93(1):83-100. http://www.mayoclinicproceedings.org/article/S0025-6196(17)30824-8/pdf
This was not so much a study as a review of current literature published in the January 2018 issue of the Mayo Clinic Proceedings under the auspices of the Mayo Foundation for Medical Education and Research. According to the review:4 Andrew Elagizi, Tobias S. Köhler, and Carl J. Lavie. “Testosterone and Cardiovascular Health.” Mayo Clin Proc. January 2018;93(1):83-100. http://www.mayoclinicproceedings.org/article/S0025-6196(17)30824-8/pdf
There is an ongoing debate in the medical community regarding the effects of testosterone on cardiovascular (CV) health. For decades, there has been conflicting evidence regarding the association of endogenous testosterone levels and cardiovascular disease (CVD) events that has resulted in much debate and confusion among health care providers and patients alike. Although there is evidence of testosterone deficiency being associated with increased mortality in multiple cohort studies, it remains unclear whether this is a causal relationship or due to a low testosterone level being a biomarker of poor overall health.5 Shores MM, Matsumoto AM. “Testosterone, aging and survival: biomarker or deficiency.” Current opinion in endocrinology, diabetes, and obesity. 2014 Jun;21(3):209-16. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313765/ Considering that CVD occurs most commonly in elderly men, and that elderly men typically have lower levels of serum testosterone and an increased chronic disease burden, it is difficult, with any surety, to assign any cause and effect to endogenous testosterone levels in regard to CV health. The evidence overall suggests that normal physiologic levels of testosterone are beneficial to the male CV system and that testosterone deficiency is associated with an unfavorable metabolic profile and increased CVD events, such as MI and mortality. Increased IMT [intima-media thickness, a measurement of the two innermost layers of an arterial wall], which has been studied as an indicator of atherosclerotic progression, is found to be associated with testosterone deficiency across multiple phenotypically distinct patient populations, including elderly men and obese adolescent females.
Low testosterone levels are associated with endothelial dysfunction in early postmenopausal women who have had their ovaries removed
 A pronounced decline in estradiol levels follows menopause, a known risk factor for cardiovascular diseases.6 Maturana MA, Irigoyen MC & Spritzer PM. “Menopause, estrogens, and endothelial dysfunction: current concepts.” Clinics vol.62 no.1 São Paulo Feb. 2007 77–86. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322007000100012 Data from experimental and clinical studies have shown a relationship between estrogen decay and endothelial dysfunction,7 Kalantaridou SN, Naka KK, Papanikolaou E, et al. “Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy.” The Journal of Clinical Endocrinology & Metabolism, Volume 89, Issue 8, 1 August 2004, Pages 3907–3913. http://academic.oup.com/jcem/article/89/8/3907/2844485 the first and most developed marker of atherosclerotic process.8 Caramori PR & Zago A. “Disfuncao endotelial e doenca arterial coronariana.” Arquivos Brasileiros de Cardiologia 2000 75 163–172. http://docplayer.com.br/33513668-Disfuncao-endotelial-e-doenca-arterial-coronariana.html Testosterone levels show a less pronounced decline (2–4 times), compared with estrogens (16 times)9 Davison SL, Bell R, Donath S, etal. “Androgen levels in adult females: changes with age, menopause, and oophorectomy.” Journal of Clinical Endocrinology and Metabolism 2005 Jul;90(7):3847-53.” http://www.ncbi.nlm.nih.gov/pubmed/15827095 because postmenopausal ovaries continue to produce testosterone. This phenomenon skews the hormone ratio in women who experience natural menopause more towards testosterone even as actual levels of testosterone are decreasing. Oophorectomized women (women who have had their ovaries removed), on the other hand, go through a different scenario, in which both estradiol and testosterone levels decline abruptly. So?
A pronounced decline in estradiol levels follows menopause, a known risk factor for cardiovascular diseases.6 Maturana MA, Irigoyen MC & Spritzer PM. “Menopause, estrogens, and endothelial dysfunction: current concepts.” Clinics vol.62 no.1 São Paulo Feb. 2007 77–86. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322007000100012 Data from experimental and clinical studies have shown a relationship between estrogen decay and endothelial dysfunction,7 Kalantaridou SN, Naka KK, Papanikolaou E, et al. “Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy.” The Journal of Clinical Endocrinology & Metabolism, Volume 89, Issue 8, 1 August 2004, Pages 3907–3913. http://academic.oup.com/jcem/article/89/8/3907/2844485 the first and most developed marker of atherosclerotic process.8 Caramori PR & Zago A. “Disfuncao endotelial e doenca arterial coronariana.” Arquivos Brasileiros de Cardiologia 2000 75 163–172. http://docplayer.com.br/33513668-Disfuncao-endotelial-e-doenca-arterial-coronariana.html Testosterone levels show a less pronounced decline (2–4 times), compared with estrogens (16 times)9 Davison SL, Bell R, Donath S, etal. “Androgen levels in adult females: changes with age, menopause, and oophorectomy.” Journal of Clinical Endocrinology and Metabolism 2005 Jul;90(7):3847-53.” http://www.ncbi.nlm.nih.gov/pubmed/15827095 because postmenopausal ovaries continue to produce testosterone. This phenomenon skews the hormone ratio in women who experience natural menopause more towards testosterone even as actual levels of testosterone are decreasing. Oophorectomized women (women who have had their ovaries removed), on the other hand, go through a different scenario, in which both estradiol and testosterone levels decline abruptly. So?
A 2016 study published in the Journal of European Endocrinology found that the absence of ovarian testosterone production in recent postmenopausal women whose ovaries had been removed was associated with negative effects on endothelial function in arterial walls and thus a greater risk of cardiovascular disease.10 Ciciliana Maila Zilio Rech, Ruth Clapauch, Maria das Gracas Coelho de Souza, and Eliete Bouskela. “Low testosterone levels are associated with endothelial dysfunction in oophorectomized early postmenopausal women.” European Journal of Endocrinology (2016) 174, 297–306. http://www.eje-online.org/content/174/3/297.full.pdf In other words, women too are affected by reductions in testosterone levels below norm. In women, however, it is more nuanced than in men as it’s less about the total level of testosterone as it is about the ratio of testosterone to estrogen in the body. In any case, dramatically lowering testosterone levels below normal in women puts them at risk.
Consumption of dairy lowers testosterone
Okay, so we’ve explored the issue of how lower levels of testosterone put you at risk for cardiovascular disease and a greater risk of dying sooner–for both men and women. Now let’s look at something that, although it might appear off topic, directly relates to the issue we’ve been talking about.
Commercial milk and dairy products are the largest sources of dietary estrogens for humans—accounting for 60-80 percent of all estrogens consumed.
Commercial milk and dairy products are the largest sources of dietary estrogens for humans–accounting for 60-80 percent of all estrogens consumed–primarily in the form of estradiol.11 Corydon Ireland. “Hormones in milk can be dangerous.” The Harvard Gazette. December 7, 2006. (Accessed 20 June 2018.) http://news.harvard.edu/gazette/story/2006/12/hormones-in-milk-can-be-dangerous/ The problem is that estradiol can lower the body’s production of testosterone–the primary male sex hormone–with the effect of raising voice pitches, increasing male breasts, and erectile dysfunction. And as bad as that is, it’s far worse than it might sound.
The protein in milk from non-pregnant cows contains about 30 pg/mL of the female hormone estrone sulphate. This increases to 151 pg/mL during early pregnancy and goes up to 1,000 pg/mL at the last stages of the pregnancy. (Note: that’s per mL, or about 1,000th of a quart.) A single glass of milk contains about 250 times that amount. Now, here’s the disturbing note of comparison. A healthy level of estradiol hormone in men is about 10-40 pg/ml. That means every glass of milk you drink is providing anywhere from 6-20 times that level–and you keep adding to that level with every glass of milk you drink, and every dairy product you consume…day after day.
That’s right. Commercial dairy cows are constantly kept pregnant to maximize milk production. Over 75 percent of commercial milk comes from pregnant cows.
Exposure to estrogen through intake of commercial milk produced from pregnant cows
 A study published in Pediatrics International found that after the intake of cow milk, both serum estrone and progesterone concentrations significantly increased, and serum luteinizing hormone, follicle-stimulating hormone, and testosterone significantly decreased in men.12 Maruyama K, Oshima T, Ohyama K. “Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows.” Pediatr Int. 2010 Feb;52(1):33-8. http://www.birdflubook.org/resources/Maruyama_2010_PI_52_33.pdf Urine concentrations of estrone, estradiol, estriol, and pregnanediol significantly increased in all adults and children. In four out of five women, ovulation occurred during the milk intake, and the timing of ovulation was similar among the three menstrual cycles. The study concluded that estrogens in milk were absorbed, and gonadotropin secretion was suppressed, followed by a decrease in testosterone secretion–not to mention the fact that the sexual maturation of prepubertal children could be affected by the ordinary intake of cow milk.
A study published in Pediatrics International found that after the intake of cow milk, both serum estrone and progesterone concentrations significantly increased, and serum luteinizing hormone, follicle-stimulating hormone, and testosterone significantly decreased in men.12 Maruyama K, Oshima T, Ohyama K. “Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows.” Pediatr Int. 2010 Feb;52(1):33-8. http://www.birdflubook.org/resources/Maruyama_2010_PI_52_33.pdf Urine concentrations of estrone, estradiol, estriol, and pregnanediol significantly increased in all adults and children. In four out of five women, ovulation occurred during the milk intake, and the timing of ovulation was similar among the three menstrual cycles. The study concluded that estrogens in milk were absorbed, and gonadotropin secretion was suppressed, followed by a decrease in testosterone secretion–not to mention the fact that the sexual maturation of prepubertal children could be affected by the ordinary intake of cow milk.
Chronic low testosterone levels in endurance trained men: the exercise-hypogonadal male condition
And it’s not just milk that drives testosterone down. A commentary that appeared in the April 2018 issue of the Journal of Biochemistry and Physiology talked about the sportsmen and women who participate in endurance events and perform a tremendous amount of exercise training.13 Hackney and Aggon. “Chronic Low Testosterone Levels in Endurance Trained Men: The Exercise-Hypogonadal Male Condition.” J Biochem Physiol 2018, 1:1. http://www.researchgate.net/profile/Anthony_Hackney/publication/324362696_Chronic_Low_Testosterone_Levels_in_Endurance_Trained_Men_The_Exercise-Hypogonadal_Male_Condition/links/5acba6de0f7e9bcd5199c065/Chronic-Low-Testosterone-Levels-in-Endurance-Trained-Men-The-Exercise-Hypogonadal-Male-Condition.pdf?origin=publication_detail For instance, it is not uncommon for a marathon or ultra-distance runner to perform 150 to 200 kilometers (93-124 miles) of intensive running per week as part of their regular training. Yes, chronic training to this extent results in positive physiological adaptations that are highly advantageous to the human body. But exercise training to this extent can also place an incredible amount of stress and strain on the athlete’s body and result in unwanted physiological responses and health problems.
One of the body’s physiological systems that is extremely sensitive to the stress of exercise training is the endocrine system. This is particularly true for the components of the endocrine system associated with the control and regulation of reproductive function. Most of the research on this topic has focused on women athletes;14 Loucks A.B. (2000) Exercise Training in the Normal Female. In: Warren M.P., Constantini N.W. (eds) Sports Endocrinology. Contemporary Endocrinology, vol 23. Humana Press, Totowa, NJ. http://link.springer.com/chapter/10.1007/978-1-59259-016-2_9 that is, specifically the study of “athletic amenorrhea (the cessation of menstrual periods)” and the “Female Athlete Triad (eating disorders, amenorrhea, and decreased bone density)”. Researchers, however, have recently begun to address the question of how exercise training affects the reproductive endocrine system in men too. Studies on men show the existence of a select group who, through their exposure to chronic endurance exercise training, have developed alterations in their reproductive hormonal profile — principally, low resting testosterone levels. Most of these men display clinically “normal” levels of testosterone but at the very low end of normal–and in some cases reach a level of “testosterone deficiency.”
Conclusion
So, what did we learn today? Well, primarily that maintaining optimized levels of free testosterone (not too much, not too little, but just the right amount) is not only essential for
- Pumping up energy levels
- Driving our desire to attack the day
- Firing the need to succeed
- Bonding us with our mates
- Fueling our sexual desires
- Elevating our levels of sexual satisfaction
- Building muscle
- Burning off fat
- Facilitating better circulation
We now know it’s also essential for maintaining cardiovascular health and preventing early death.
And considering all the things that work against maintaining that optimized level–age, xenoestrogens, too much exercise, and diet (especially too much dairy)–actively intervening and supplementing to maintain optimum levels of free testosterone make all the sense in the world.
References
| ↑1 | Stine A Holmboe1, Niels E Skakkebæk, Anders Juul, et al. “Individual testosterone decline and future mortality risk in men.” Eur J Endocrinol January 1, 2018 178 121-128. http://www.eje-online.org/content/178/1/121.abstract |
|---|---|
| ↑2 | Thiago Gagliano-Jucá, Thomas G Travison, Philip W Kantoff, et al. “Androgen Deprivation Therapy Is Associated With Prolongation of QTc Interval in Men With Prostate Cancer.” Journal of the Endocrine Society, Volume 2, Issue 5, 1 May 2018, Pages 485–496. http://academic.oup.com/jes/article/2/5/485/4980315 |
| ↑3 | Antonopoulos AS, Antoniades C. “Mechanisms of testosterone deficiency-related endothelial dysfunction.” Hellenic J Cardiol. 2018 Jun 8. pii: S1109-9666(18)30168-4. http://www.sciencedirect.com/science/article/pii/S1109966618301684?via%3Dihub |
| ↑4 | Andrew Elagizi, Tobias S. Köhler, and Carl J. Lavie. “Testosterone and Cardiovascular Health.” Mayo Clin Proc. January 2018;93(1):83-100. http://www.mayoclinicproceedings.org/article/S0025-6196(17)30824-8/pdf |
| ↑5 | Shores MM, Matsumoto AM. “Testosterone, aging and survival: biomarker or deficiency.” Current opinion in endocrinology, diabetes, and obesity. 2014 Jun;21(3):209-16. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313765/ |
| ↑6 | Maturana MA, Irigoyen MC & Spritzer PM. “Menopause, estrogens, and endothelial dysfunction: current concepts.” Clinics vol.62 no.1 São Paulo Feb. 2007 77–86. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322007000100012 |
| ↑7 | Kalantaridou SN, Naka KK, Papanikolaou E, et al. “Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy.” The Journal of Clinical Endocrinology & Metabolism, Volume 89, Issue 8, 1 August 2004, Pages 3907–3913. http://academic.oup.com/jcem/article/89/8/3907/2844485 |
| ↑8 | Caramori PR & Zago A. “Disfuncao endotelial e doenca arterial coronariana.” Arquivos Brasileiros de Cardiologia 2000 75 163–172. http://docplayer.com.br/33513668-Disfuncao-endotelial-e-doenca-arterial-coronariana.html |
| ↑9 | Davison SL, Bell R, Donath S, etal. “Androgen levels in adult females: changes with age, menopause, and oophorectomy.” Journal of Clinical Endocrinology and Metabolism 2005 Jul;90(7):3847-53.” http://www.ncbi.nlm.nih.gov/pubmed/15827095 |
| ↑10 | Ciciliana Maila Zilio Rech, Ruth Clapauch, Maria das Gracas Coelho de Souza, and Eliete Bouskela. “Low testosterone levels are associated with endothelial dysfunction in oophorectomized early postmenopausal women.” European Journal of Endocrinology (2016) 174, 297–306. http://www.eje-online.org/content/174/3/297.full.pdf |
| ↑11 | Corydon Ireland. “Hormones in milk can be dangerous.” The Harvard Gazette. December 7, 2006. (Accessed 20 June 2018.) http://news.harvard.edu/gazette/story/2006/12/hormones-in-milk-can-be-dangerous/ |
| ↑12 | Maruyama K, Oshima T, Ohyama K. “Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows.” Pediatr Int. 2010 Feb;52(1):33-8. http://www.birdflubook.org/resources/Maruyama_2010_PI_52_33.pdf |
| ↑13 | Hackney and Aggon. “Chronic Low Testosterone Levels in Endurance Trained Men: The Exercise-Hypogonadal Male Condition.” J Biochem Physiol 2018, 1:1. http://www.researchgate.net/profile/Anthony_Hackney/publication/324362696_Chronic_Low_Testosterone_Levels_in_Endurance_Trained_Men_The_Exercise-Hypogonadal_Male_Condition/links/5acba6de0f7e9bcd5199c065/Chronic-Low-Testosterone-Levels-in-Endurance-Trained-Men-The-Exercise-Hypogonadal-Male-Condition.pdf?origin=publication_detail |
| ↑14 | Loucks A.B. (2000) Exercise Training in the Normal Female. In: Warren M.P., Constantini N.W. (eds) Sports Endocrinology. Contemporary Endocrinology, vol 23. Humana Press, Totowa, NJ. http://link.springer.com/chapter/10.1007/978-1-59259-016-2_9 |












Two questions:
Two questions:
First, I understand from my doctor that at near 70 years of age, keeping my testosterone serum levels around 800-1000 is good. You did not mention levels in your letter.
Second, I have read and informed that as we age we need higher testosterone levels than those younger.
Your thoughts please……
This newsletter was focused
This newsletter was focused on current studies. If you want to know more about the ins and outs concerning testosterone, then you should check out this newsletter. https://jonbarron.org/what-men-and-women-need-know-about-testosterone
as a male over 60 with b h p,
as a male over 60 with b h p, would it not be dangerous to elevate testosterone levels?
Chris
You might find this article
You might find this article informative. https://jonbarron.org/article/30000-mile-tune
I fit your profile 100%
I fit your profile 100%
70 years of age
diabetic 1994 – 2008 thanks to glucator
2 small heart attacks – 2017 jan. and june
due to dailysis commencing 2016.Started
taking k-2 mk7 – no more clogged arteries
i haven’t had an erection since 2006
using viagra 2004-2007
Jon,
Jon,
Another excellent job. This is amazing how our society promotes dairy in so many foods! A learning curve is needed. And fast!
If low testosterone levels
If low testosterone levels are a risk factor for cardiovascular disease, then statins – prescribed to lower cholesterol – which is required for the synthesis of steroidogenic hormones – would now appear to actually raise rather than lower the risk for heart disease. Is this an accurate conclusion??
Statin drugs can definitely
Statin drugs can definitely lower cholesterol levels. But whether that adds even a single day to your life is open to debate—even within the medical community. https://jonbarron.org/article/statin-drugs-%E2%80%93-jama-debate
Such an awareness creation
Such an awareness creation blog you have written. Talking about the Testosterone levels in the body and their effects in taking up commercial milk in our day-to-day life. Helpful Share.