Last month, I wrote about how NY Attorney General Eric Schneiderman’s February attack on the Gang of Four (GNC, Target, Walmart, and Walgreen’s) for selling “allegedly” contaminated and mislabeled supplements was based on faulty information derived from a DNA test not appropriate for the task at hand. Surprisingly, several people wrote in to defend him saying that he was a good man who meant well and had just made a mistake in this case. In other words, I should give him the benefit of the doubt. Unfortunately, I had a problem with that because once the error of his testing methodology was revealed to him, he didn’t acknowledge the error and apologize to the companies in question. Instead, in an effort to hide his error and make himself look good, he squeezed GNC into agreeing to incorporate DNA testing in the future–when it might be ready for testing ingredients but not finished products. The bottom line is that in order to cover his mistake, he let stand the original false impression that most supplements are contaminated and mislabeled. And this has led to a large number of vicious media attacks on the supplement industry–all based on a lie. For example:
- Salon: “Massive Herbal-Supplement Scam Uncovered: Walmart, Target, GNC Accused of Selling Bogus Products”1 Joanna Rothkopf. “Massive Herbal-Supplement Scam Uncovered: Walmart, Target, GNC Accused of Selling Bogus Products.” Salon. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.salon.com/2015/02/03/Walmart_target_walgreens_gnc_accused_of_selling_scam_herbal_supplements/
- CBS News: “Herbal supplements filled with fake ingredients, investigators find”2 “Herbal supplements filled with fake ingredients, investigators find.” CBS News. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.alternet.org/corporate-accountability-and-workplace/massive-herbal-supplement-scam-uncovered-Walmart-target-gnc” CBS News. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.cbsnews.com/news/herbal-supplements-targeted-by-new-york-attorney-general/
- CNN Money: “Wal-mart, Target and others under fire for selling bogus supplements”3 Gregory Wallace. “Wal-mart, Target and others under fire for selling bogus supplements.” CNN Money. Feb 4, 2015. (Accessed 12 Jun 2015.) http://money.cnn.com/2015/02/03/news/herbal-supplements-Walmart-target/index.html
- ABC Action News: “Walmart, GNC, Target and Walgreens under fire for selling bogus supplements.”4 Associated Press. “Walmart, GNC, Target and Walgreens under fire for selling bogus supplements.” ABC Action News. Feb 3 2015. (Accessed 12 Jun 2015.) http://www.abcactionnews.com/news/national/Walmart-gmc-target-and-walgreens-under-fire-for-selling-bogus-supplements
- Fox News Insider. “New York AG: Many Popular Store-Brand Supplements Are Phony.”5 “New York AG: Many Popular Store-Brand Supplements Are Phony.” Fox News Insider. Feb 3, 2015. (Accessed 12 Jun 2015.) http://insider.foxnews.com/2015/02/03/new-york-ag-Walmart-target-gnc-walgreens-supplements-are-phony-contaminated
Well, it turns out, I was right not to give AG Schneiderman the benefit of the doubt. The false attack against the Gang of Four was not a one-off from which he was forced to cover his behind, but merely the opening salvo in what appears to be a prolonged fight to the death–with truth the ultimate victim.
A Quick Review of Schneiderman’s Initial Attack–the First Event
In early February, New York Attorney General Eric T. Schneiderman announced in a press release that his office had sent cease and desist letters to four major retailers–GNC, Target, Walmart, and Walgreens–for allegedly selling store brand herbal supplements in New York that either did not contain the herbs listed on the label, or which were found to contain contaminating ingredients that were not listed on the labels. The AG’s office used a form of testing known as DNA Barcoding to arrive at these results.
Unfortunately, DNA Barcoding turns out to be useless for testing herbal extracts or tinctures.
The reason is simple, as anyone familiar with DNA Barcoding and plants can tell you: there is no plant matter left in an extract or tincture, except perhaps trace amounts of any sediment that might be found at the bottom of the bottle. Extracts and tinctures are pretty much DNA free by definition. What they contain are concentrated bioactive ingredients, not plant matter. DNA is only present in the plant matter, not the extract. To put it in simple terms, an herbal extract of Echinacea might be extremely concentrated and extremely potent, but show no DNA. There are, however, tests used as part of normal current Good Manufacturing Practices (cGMP) that can identify whether the appropriate biochemicals for a particular herb are present or not, but DNA Barcoding is not one of them.
The other problem is that DNA Barcoding, although it may be able to tell you whether or not the DNA of a particular plant is present or not, can’t tell you how much of that plant is present. If you harvest a field of a given crop and there are a handful of weeds in that harvest, DNA Barcoding will show the presence of the DNA from those weeds, even if it represents .001 percent of the total. The bottom line is that with any natural crop that you grow outdoors, there is going to be some contaminating plant matter in that crop, but usually of such a low percentage that it has zero impact on the human body.
Also, once you bring that crop into the warehouse for additional processing, small levels of additional contaminants are likely to be introduced. Keep in mind that the FDA actually understands this and sets guidelines in their Defect Levels Handbook detailing acceptable levels of contamination, or as it explains in the handbook, ” … because it is economically impractical to grow, harvest, or process raw products that are totally free of non-hazardous, naturally occurring, unavoidable defects.”6 “Defect Levels Handbook.” FDA. May 1998. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/SanitationTransportation/ucm056174.htm And understand, it’s not just extraneous plant matter the FDA is talking about; it’s insect parts, rodent hairs, mouse feces, mold, mildew, fly eggs, and maggots. Here are three examples from the handbook.
- Cocoa beans are allowed up to 4% mold, 4% insect-infested, and 10 mg or more of mammalian excreta per pound.
- Peanut butter averages 30 or more insect fragments per 100 grams.
- The average mold count on all berries (canned and frozen) is 60% or more, and 4 or more insect larvae and 10 or more whole insects per 500 grams.
(Sorry about that. You’ll probably never look at your PB and J sandwich the same way again, but that’s reality in the food industry.)
For Eric Schneiderman to present trace plant matter contamination in food-based, herbal supplements as a shocking revelation, when there’s actually an FDA guidebook explaining allowable levels of contamination in virtually every food we eat is calculatedly lacking in candor.
But the biggest credibility problem Schneiderman faces regarding DNA Barcode testing is that as soon as GNC signed its agreement with the AG’s office, Schneiderman rescinded the cease and desist order on their “phony” products and authorized their immediate release for sale to the public. This can mean only one of two possible scenarios, and neither one speaks well of the AG’s office.
 The DNA Barcode testing was indeed accurate–meaning that the products really didn’t contain the labeled ingredients and were indeed laced with contaminating ingredients–and yet the Attorney General cynically agreed to release those same tainted products for sale to the public in a quid pro quo exchange with GNC. That would be deeply disturbing–and possibly illegal. Investigators of political corruption don’t like quid pro quo deals.
The DNA Barcode testing was indeed accurate–meaning that the products really didn’t contain the labeled ingredients and were indeed laced with contaminating ingredients–and yet the Attorney General cynically agreed to release those same tainted products for sale to the public in a quid pro quo exchange with GNC. That would be deeply disturbing–and possibly illegal. Investigators of political corruption don’t like quid pro quo deals.- The results of the DNA Barcode testing were NOT accurate because DNA Barcode testing is indeed not suitable for the task at hand. However, to avoid acknowledging an embarrassing mistake, the AG squeezed GNC to sign the agreement, removed the cease and desist order, which never should have been issued in the first place, and then unconscionably continued to present to the public that they were in danger from mislabeled, contaminated product as offered by almost all supplement companies. And as payback to Walmart, Walgreens, and Target, because they did not sign his extortion agreement, he refused to remove the cease and desist from their products even though they too were falsely convicted by the same inappropriate testing methodology. In fact, subsequent events prove that this was the more likely scenario. Incidentally, in some ways, this is even more cynical and more disturbing than if the DNA testing had been accurate.
The Second Event
In April, Schneiderman continued his disingenuous assault on the supplement industry by recruiting 13 other Attorneys General (let’s call them the 13 Ghosts) to ghost sign a letter to congressional leaders that he had crafted asking for a “comprehensive inquiry” into the still largely unregulated industry.7 “A Communication from the Chief Legal Officers of the Following States and Territories: Connecticut * District of Columbia * Hawaii * Idaho Indiana * Iowa * Kentucky * Massachusetts * Mississippi New Hampshire * New York * Northern Mariana Islands Pennsylvania * Rhode Island. State Attorneys General. April 2, 2015. (Accessed 10 Jun 2015.) http://www.ag.ny.gov/pdfs/Final%20Letter%20Re%20Herbal%20Supplements.pdf The letter also asked lawmakers to consider “a more robust oversight role” for the U.S. Food and Drug Administration.
“When consumers take an herbal product, they should be able to do so with full knowledge of what is in that product and confidence that every precaution was taken to ensure its authenticity and purity.”
The letter cited Schneiderman’s earlier investigation based on DNA Barcoding that concluded that 80% of the herbal products it obtained from the Gang of Four were contaminated with wheat, legumes, and other ingredients dangerous to consumers with allergies and did not contain any of the herbs listed on their labels. Not surprisingly, the letter did not cite the fact that Schneiderman had subsequently released GNC’s versions of those products for sale with no further testing–merely as a reward for signing his PR agreement. But again, if they were contaminated, how could he release them for sale? And if they weren’t contaminated (in other words, if DNA Barcode testing produced incorrect results because it doesn’t work for extracts), then how could he cynically use those false results as justification for continuing his witch hunt against the supplement industry? Just asking!
The Third Event
In a letter dated May 26, Schneiderman teamed up with Indiana Attorney General, Greg Zoeller, jointly sending a letter written by Schneiderman to Dr. Stephen Ostroff, the acting commissioner of the Food and Drug Administration (FDA).8 E Schneiderman and G Zoeller. “Letter to Dr. Stephen Ostroff.” Office of the Attorney General, State of New York. 26 May 2015. (Accessed 12 Jun 2015.) http://www.oag.state.ny.us/pdfs/Letter_to_FDA_5_26_2015.pdf The letter essentially repeats the claims made in the April congressional letter claiming that the supplement industry is getting away with murder and “urges” the FDA to “overhaul” its oversight of the dietary supplement industry. To quote from the letter:
“The health and safety of the tens of millions of Americans who take supplements every single day will continue to be jeopardized until these lax regulations are improved. I urge the FDA to use every tool at its disposal to protect consumers in New York and across the country from the unnecessary risk of tainted supplements.”
Specifically, the letter asks the FDA to address four “flaws” that Schneiderman claims to have identified in cGMP:
 First, in promulgating cGMP rules, the FDA elected not to cover ingredient suppliers, which compromises the integrity of the supply chain. Also, many are located overseas and beyond the reach of effective enforcement actions, said the AGs.
First, in promulgating cGMP rules, the FDA elected not to cover ingredient suppliers, which compromises the integrity of the supply chain. Also, many are located overseas and beyond the reach of effective enforcement actions, said the AGs.- Second, the cGMP rules put flexibility ahead of quality and safety concerns, allowing manufacturers to set their own label specifications and then choosing their own test for confirming label claims. The tests used by most manufacturers often cannot distinguish genuine products from chemically similar natural and synthetic compounds.
- Third, cGMP rules do not require manufacturers to engage in any confirmatory testing to ensure that supplements are free of common allergens, even where products are marketed as containing no allergens (e.g. ‘gluten-free’).
- “Finally, the cGMP rules fail to define the key terms applied to dietary supplements or require manufacturers to disclose to consumers how those terms apply to a given product. These terms are poorly understood and manufacturers use them in ambiguous and conflicting ways. For example, with herbal supplements, the industry applies the term “extract” to a spectrum of products–from minimally processed plants to highly purified chemicals. When consumers buy a “natural” product–which typically features a root, a leaf, a flower, or a piece of bark on the label–they do not reasonably expect to receive a heavily processed chemical. Yet, more often than not, this is what they get.”
Unfortunately, all of these so called “flaws” are misleading at best and cynically mendacious at worst. Let’s deal with them one by one.
1. cGMP Does Not Apply to Ingredient Suppliers
Messrs. Schneiderman and Zoeller are absolutely correct–but also absolutely deceitful–in this assertion. Yes, it is true that herbal ingredient suppliers are not covered under cGMP, but that’s because most of the herbs they sell are classified as foods. Think garlic, as an example–used as both a food and in herbal formulations. The key here is that although foods are not covered by cGMP, they are absolutely covered by the Food Safety Modernization Act (FSMA).9 “Full Text of the Food Safety Modernization Act (FSMA).” FDA. (Accessed 10 June 2015.) http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm247548.htm The act specifies requirements for testing foods/ingredients by accredited laboratories, as well as requirements for identifying and dealing with potential allergens in those foods/ingredients. To claim that ingredient suppliers are outside cGMP regulations is either ignorant or disingenuous. Take your pick. As Steve Mister of the Council for Responsible Nutrition says, “FSMA is vastly overhauling all food ingredient suppliers, including dietary supplement ingredient suppliers.”10 Stephen Daniels. “NY and IN AG urge FDA to ‘overhaul’ supplement regulations.” Nutra Ingredients. 3 June 2015. (Accessed 11 Jun 2015.) http://www.nutraingredients-usa.com/Regulation/NY-and-IN-AG-urge-FDA-to-overhaul-supplement-regulations
2. Manufacturers Tests Are Inadequate
The claim that the tests used by most manufacturers cannot distinguish genuine products from chemically similar natural and synthetic compounds is patently false. Any cGMP compliant manufacturer will be set up with an in-house lab equipped with instruments such as a high performance liquid chromatograph, moisture determination meter, Fourier transform infrared spectrometer, near infrared spectroscopy, atomic absorption spectrophotometer, gas chromatograph, etc. These tests can not only tell whether a bag of ground herb is what it purports to be, but whether it is the right genus and whether the key bioactives are present in sufficient quantity. Let’s look at just one of these tests, infrared spectroscopy, in a little more detail.
I nfrared spectroscopy is an analytical technique used primarily to identify carbon-based materials. It can be utilized to identify components of an unknown mixture in solid and liquid forms. Within the infrared wavelengths of light, there are three general groupings: near, mid and far infrared light. The different groupings reveal different things about an ingredient. The mid frequencies, for example, are useful for revealing any of a variety of adulteration problems. The near frequencies (NIR testing), because of their ability to penetrate far into a sample, are very useful in exploring bulk material with little or no sample preparation. NIR can quickly determine whether or not you have the ingredient you want simply by matching the waveform of the tested item to a known waveform of the same ingredient in your computerized database. Its value in testing supplements is that the waveform not only reveals the genus of an herbal ingredient being tested, but also its particular species, and even what part of the plant is present (root, bark, leaf, etc.)–something DNA Barcoding cannot do. Here are the waveforms for the three primary species of Echinacea for both leaves and roots. (Incidentally, when it comes to Echinacea, the roots are more potent than the leaves, so knowing which part of the plant you’re dealing with is important.) Notice how the waveforms are both similar (as one would expect since they all are of Echinacea) and different (reflecting different species of Echinacea and different parts of the plant).
nfrared spectroscopy is an analytical technique used primarily to identify carbon-based materials. It can be utilized to identify components of an unknown mixture in solid and liquid forms. Within the infrared wavelengths of light, there are three general groupings: near, mid and far infrared light. The different groupings reveal different things about an ingredient. The mid frequencies, for example, are useful for revealing any of a variety of adulteration problems. The near frequencies (NIR testing), because of their ability to penetrate far into a sample, are very useful in exploring bulk material with little or no sample preparation. NIR can quickly determine whether or not you have the ingredient you want simply by matching the waveform of the tested item to a known waveform of the same ingredient in your computerized database. Its value in testing supplements is that the waveform not only reveals the genus of an herbal ingredient being tested, but also its particular species, and even what part of the plant is present (root, bark, leaf, etc.)–something DNA Barcoding cannot do. Here are the waveforms for the three primary species of Echinacea for both leaves and roots. (Incidentally, when it comes to Echinacea, the roots are more potent than the leaves, so knowing which part of the plant you’re dealing with is important.) Notice how the waveforms are both similar (as one would expect since they all are of Echinacea) and different (reflecting different species of Echinacea and different parts of the plant).
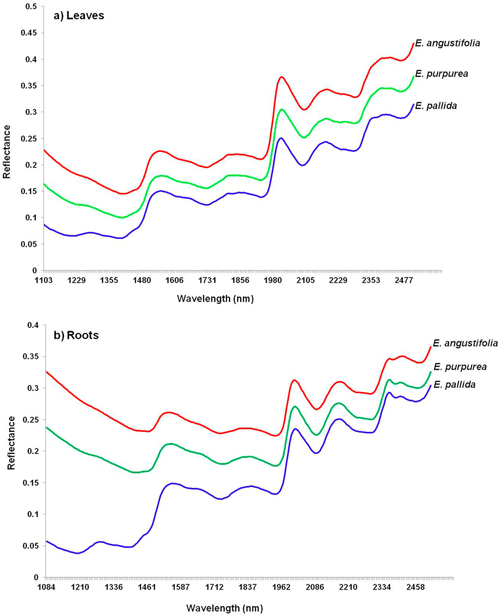
The bottom line is that current testing methodologies used by cGMP manufacturers are more than up to the task of determining what’s in a supplement–and far more suitable than DNA Barcoding for the task at hand.
3. Supplements Contain Allergens
When it comes to allergens, supplement ingredients are governed by the same regulations that govern foods. Allergens beyond minimal levels as determined by the FDA must be identified on the label. In addition, cGMP requires manufacturers to identify all ingredients present in the final product.
4. Consumers Do Not Understand Extracts
Of all the concerns expressed in Schneiderman’s most recent letter, this is the silliest and most insulting–and can quickly be put paid to.
Here is a picture of a vanilla bean and a bottle of vanilla extract. Not one person–except, perhaps, an attorney general–thinks these two are the same thing. Consumers understand that the extract is made from vanilla beans, but they also know that the extract is not the same as the vanilla bean, that it is stronger than the original bean and contains liquid that is not found in the bean–and that’s exactly why they buy it and use it.

They also know that natural vanilla extract is not the same as synthetic vanillin. Current labeling laws require that the manufacturer note the use of synthetic, chemical versions of herbs on the label.

And even when used as one of several ingredients, current labeling laws require the manufacturer to identify the use of any synthetic, chemical herbs in the list of ingredients.
There is no attempt to deceive. No one is deceived.
Now let’s look at Echinacea. Here is a picture of an Echinacea plant and a bottle of Echinacea extract. Not one person–again, except, perhaps, an attorney general–thinks these two are the same thing. People understand that the extract is made from parts of the plant, that the extract is stronger than the original plant and contains liquid that is not found in the plant. Again, that’s why they buy it and use it–because it’s concentrated.

There is nothing deceptive here. The difference between the Echinacea plant and the extract made from it is obvious to everyone, except 14 attorneys general. And do you notice the remarkable similarity of this Echinacea picture to the vanilla example we looked at earlier? That’s not an accident. Both involve plants as the initial raw ingredient, and the process of making an extract from those plants is identical in both cases. If you think the Echinacea extract is a “heavily processed chemical,” then you must make the same assessment of the vanilla extract. And yet, why is it that I feel Schneiderman is not going to go after vanilla extract?
The bottom line, as we have seen, is that when Eric Schneiderman says that “the tests used by most manufacturers often cannot distinguish genuine products from chemically similar natural and synthetic compounds,” he is categorically incorrect. Current tests used by cGMP compliant supplement manufacturers can indeed tell the difference. Amazingly, stunningly, bafflingly, the one test that can’t do that is the test Schneiderman wants to use: DNA Barcoding. As we have explained, the reason DNA Barcoding can’t detect any genetic material in extracts is because there isn’t any left in the extract. All of the DNA stays with the non-active plant matter that is filtered off after the extraction is complete. But instead of simply acknowledging that he used the wrong test for the task at hand and chose the wrong person to conduct that test (a specialist in reptile DNA who had no idea that when you make a plant extract you remove the plant DNA), he’s decided to blame herbal extracts for his error…simply because they’re herbal extracts. As Steve Mister said: “To begin with, he [Schneiderman] continues to hold out the tests he reported in February as if there is any validity to them. Experts from inside and outside the industry agree that the concept of DNA barcoding of extracts is inappropriate and misinformed…Even if the test was valid, his results are so random and all over the place that they say more about the inability of the testers than the products themselves.”11 Stephen Daniels
Conclusion 1
Do I really believe that Eric Schneiderman and the 13 AG’s he got to sign his letter are clueless and have no idea what they’re doing? Most likely the 13 who signed the April letter to Congress are indeed clueless. If I were to guess, I think they merely agreed to sign on with Schneiderman because it seemed like a good idea at the time–a no risk way to score some points with their constituents by appearing to be “protecting” them from the villainous supplement industry. It may not work out that way.
Little did they know that the letter that they signed was based on lies and deception and could ultimately cost their constituents a great deal of money. (We’ll be exploring that in a moment.)
Schneiderman, though, is not clueless. He knows exactly what’s going on. He read some reports in the paper about supplements being sold that were not what they were purported to be and saw a chance to advance his career. Nothing wrong with that; everyone’s entitled. He then read about DNA Barcoding and mistakenly thought it would be a “cutting edge” way to analyze some supplements and see if he could build a case. Hey! Anyone can make a mistake; still not a problem. He then hired the wrong person to conduct the tests–a reptile specialist who had no understanding of herbs or what supplement processing does to DNA. The mistakes are starting to add up, but still they’re mistakes.
Then he made his results public and used the power of his office to send cease and desist letters to GNC, Walgreens, Walmart, and Target telling them to stop selling a range of supplements that he had tested. This was a really big mistake, bordering on abuse of power, but it was still a mistake–and still recoverable!
But finally, he crossed the line. Subsequently learning that DNA Barcoding had produced faulty results and that his assumptions were faulty, he arrived at a moral crossroads–and took the amoral path. Instead of just copping to the mistake and releasing the Gang of Four to sell their products, he chose to double down on his error–to keep it hidden. He got GNC to sign off on his PR agreement. In exchange, he rescinded the cease and desist letter against GNC, but not against the three other companies. As I said earlier, this can mean only one of two things, and neither one speaks well of Schneiderman’s motives. Either:
- The DNA Barcode testing was indeed accurate–meaning that the products didn’t contain the labeled ingredients and were indeed dangerously laced with contaminating ingredients–and yet the Attorney General cynically agreed to release those tainted products for sale to the public. But as we now know, DNA Barcoding does not work as a test for supplements, which means that door number two is the far more likely scenario.
- The results of the DNA Barcode testing were not accurate because DNA Barcode testing is not suitable for the task at hand, and Schneiderman now knows this. But to preserve the appearance of credibility, he got GNC to participate in his PR event in exchange for releasing their product that he now knew to be perfectly safe and correctly labeled. Nevertheless, he cynically continued to present to the public that they were in danger from mislabeled contaminated, supplements.
But even worse, he has not released the Walmart, Walgreens, and Target products even though they too were judged by the same faulty test. If the GNC product is safe for release with no further testing, then so are the Walmart, Walgreens, and Target products. This is not only cynical; it is an unconscionable abuse of power. I think it is more than noteworthy that at this point in time, four months after Schneiderman issued his cease and desist letters, only GNC has agreed to buckle. One can only wonder what the other three are planning.
Conclusion 2
Let’s just consider for a moment what it means if Schneiderman and his 13 Ghosts get their way and DNA Barcode testing becomes mandatory for all ingredients used in herbal supplements. If Schneiderman is right, it further guarantees the quality of supplements sold, right? And if it he isn’t, then what’s the problem. If there’s no harm, there’s no foul, right? Unfortunately, there is harm, and there is potentially a huge foul. Consider:
Current testing used in cGMP manufacturing fully guarantees that ingredients are what they’re supposed to be. And current labeling laws require that labels accurately reflect what’s in supplements. Now if a company refuses to obey the law because the required testing is too costly and decides to sell you contaminated product, that’s a different story. But that has nothing to do with current tests not being up to the task at hand. It has to do with a handful of companies being willing to break the law because current testing costs more than they want to spend. Adding more tests will not create more compliance, but in fact, is likely to create less compliance as costs go up and more companies choose to sell products without paying those costs. The bottom line is that the tests currently in place and the laws currently written are sufficient to the task–if they are followed. Compliance, not more testing, is the answer. When you think about it, this is no different than what you see in the food industry where we learn about adulterated and contaminated foods being recalled all the time. Again, the law is specific, but not everyone complies. The answer isn’t more regulation, but better compliance.
Then we need to consider, if useless testing is added to the process of making your supplements, who will pay that cost? Why you will, of course! Any additional costs will be passed onto you by manufacturers–and, as we’ve seen, you will not receive one single benefit from these added costs.
Right now, you might be thinking to yourself, “What do I care? I don’t buy that many supplements. What does it matter if their cost goes up a few cents?” But here’s where things start to get very interesting.
If we implement mandatory DNA Barcode testing for herbal ingredients, what does that mean for foods in general? Remember, herbal ingredients, as with all other foods, are governed by the Food Safety Modernization Act. If you require DNA Barcode testing for herbal ingredients, aren’t you also requiring it for every single food item sold in the store? Think about this for a moment. Many herbs/foods actually serve dual purposes–that is, they are sold both as foods and supplements. Examples off the top of my head include: garlic, oregano, olive leaf, apple pectin, oat fiber, licorice, peppermint, orange peel, raspberries, pomegranates, beets, seaweed, grape skins, grape seeds, cayenne, broccoli sprouts, cilantro, chlorella, ginger, avocado/soy, horseradish, apple cider vinegar, pineapple (bromelain), papain, cinnamon, cardamom, clove, black pepper, parsley, and fenugreek. That means that if the AG’s have their way, the cost of every single food you buy will eventually go up–again, for no benefit but to advance the careers of some opportunistic politicians. Are you starting to find things a bit more interesting?
And then there’s the problem that the FDA has, to date, only authorized one company to do DNA Barcode testing on foods and herbs–incidentally, not the one the NYAG used for his tests. (In September 2014, the FDA awarded AuthenTechnologies an exclusive contract for up to three years to develop a collection of validated reference DNA sequences and reference materials for a variety of botanical species.) If the AG’s have their way and the FDA ends up insisting on mandatory testing for all herbal ingredients, the entire supplement industry would come to a standstill as every ingredient supplier would have to run their herbs through this one company–and that company is still working on developing its methodologies. That would drive costs through the roof, as well as create staggering backorder problems for many supplements. But again, if you’re not a supplement user, what does it matter? Well remember, unless the FDA exempts the food supply from DNA Barcoding–and how can they do that because of the massive crossover between foods and herbal supplements–the entire food supply of the country would now have to run through one company for testing. Forget astronomical costs, what do you think that’s going to mean for the availability of some of your favorite foods?
And what do you think that would mean for the political career of any politician whose name was associated with those increased costs? I’m guessing that it would be decidedly shorter than it might have been otherwise.
The bottom line is that unless Schneiderman and his 13 Ghosts don’t want to be known as the people who brought the US food supply to its knees and drove prices through the roof, they need to stand down. Current testing and labeling in the supplement industry is more than adequate for the task at hand. As with the food industry and the pharmaceutical industry, for that matter, what’s needed is better compliance.
Final thought. You’ve really got to think that it means something that Target, Walmart and Walgreens didn’t follow in GNC’s footsteps and roll over for the NYAG. It’s been four months now and nothing. What are they planning? I would guess that legal action against the State of New York is at least being considered by top management. How delicious that would be if they went through with it. And you’ve got to believe the AG would have a major problem defending his release of GNC’s products for sale. That would stand as proof in most courts that he knew he was wrong to issue the cease and desist in the first place, that all of the products sold by the Gang of Four were what they said they were, and yet he still restricted the trade of three major corporations–purely for political reasons. Res ipsa loquitur. The thing speaks for itself!

References
| ↑1 | Joanna Rothkopf. “Massive Herbal-Supplement Scam Uncovered: Walmart, Target, GNC Accused of Selling Bogus Products.” Salon. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.salon.com/2015/02/03/Walmart_target_walgreens_gnc_accused_of_selling_scam_herbal_supplements/ |
|---|---|
| ↑2 | “Herbal supplements filled with fake ingredients, investigators find.” CBS News. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.alternet.org/corporate-accountability-and-workplace/massive-herbal-supplement-scam-uncovered-Walmart-target-gnc” CBS News. Feb 3, 2015. (Accessed 12 Jun 2015.) http://www.cbsnews.com/news/herbal-supplements-targeted-by-new-york-attorney-general/ |
| ↑3 | Gregory Wallace. “Wal-mart, Target and others under fire for selling bogus supplements.” CNN Money. Feb 4, 2015. (Accessed 12 Jun 2015.) http://money.cnn.com/2015/02/03/news/herbal-supplements-Walmart-target/index.html |
| ↑4 | Associated Press. “Walmart, GNC, Target and Walgreens under fire for selling bogus supplements.” ABC Action News. Feb 3 2015. (Accessed 12 Jun 2015.) http://www.abcactionnews.com/news/national/Walmart-gmc-target-and-walgreens-under-fire-for-selling-bogus-supplements |
| ↑5 | “New York AG: Many Popular Store-Brand Supplements Are Phony.” Fox News Insider. Feb 3, 2015. (Accessed 12 Jun 2015.) http://insider.foxnews.com/2015/02/03/new-york-ag-Walmart-target-gnc-walgreens-supplements-are-phony-contaminated |
| ↑6 | “Defect Levels Handbook.” FDA. May 1998. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/SanitationTransportation/ucm056174.htm |
| ↑7 | “A Communication from the Chief Legal Officers of the Following States and Territories: Connecticut * District of Columbia * Hawaii * Idaho Indiana * Iowa * Kentucky * Massachusetts * Mississippi New Hampshire * New York * Northern Mariana Islands Pennsylvania * Rhode Island. State Attorneys General. April 2, 2015. (Accessed 10 Jun 2015.) http://www.ag.ny.gov/pdfs/Final%20Letter%20Re%20Herbal%20Supplements.pdf |
| ↑8 | E Schneiderman and G Zoeller. “Letter to Dr. Stephen Ostroff.” Office of the Attorney General, State of New York. 26 May 2015. (Accessed 12 Jun 2015.) http://www.oag.state.ny.us/pdfs/Letter_to_FDA_5_26_2015.pdf |
| ↑9 | “Full Text of the Food Safety Modernization Act (FSMA).” FDA. (Accessed 10 June 2015.) http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm247548.htm |
| ↑10 | Stephen Daniels. “NY and IN AG urge FDA to ‘overhaul’ supplement regulations.” Nutra Ingredients. 3 June 2015. (Accessed 11 Jun 2015.) http://www.nutraingredients-usa.com/Regulation/NY-and-IN-AG-urge-FDA-to-overhaul-supplement-regulations |
| ↑11 | Stephen Daniels |












Well done Jon.
Well done Jon.
Thank you.
Way to tell it like it is, and provide food for thought to the big 4s attorneys. Too much abuse of power by this attorney general – I do hope someone lets him know in court how it really is. Its sounds like a strong conspiracy to make money on rididulous further monopolized regulations. Extract this attorney general and lets check his DNA.
Its hard not to argue with
Its hard not to argue with such passionate words and clear thinking. I remain optimistic that Eric will go down in a blaze undignified glory; as the walls and his demons close-in all around him. You took a complex subject and simplified it. Thanks for your understanding and insight.
Kudos, Jon. I could actually
Kudos, Jon. I could actually finally understand whats going on with this case. Insightful and well written.
Well done John. A riposte
Well done John. A riposte like no other – hope the gang of four (three) give him a drubbing in court.
I buy my vanilla from The
I buy my vanilla from The Vanilla Queen. It’s expensive but real and strong vanilla flavor.
Superbly written article. You
Superbly written article. You certainly simplify things for those of us less informed. Many thanks for everything you do.
How hard is it to say “Oops,
How hard is it to say “Oops, I made a mistake(s)”. This need to save face is causing a lot of unnecessary problems.
If these guys have such a strong need to be boost their careers, become ‘heros’ and save us from tainted supplements, why don’t they turn their attention instead to actual food and companies such as giant Monsanto and the GMO boom? (rhetorical question, I already know the answer).
I am hoping that Walmart, Walgreens, and Target are going to sue so this all comes out in the wash and the AGs get what they deserve, to be outed as dishonest and abusing their power. Won’t be the first time a gov’t agency has been accused of being corrupt.
I don’t even know what to think about GNC anymore after reading this..
Thank you for such an indepth
Thank you for such an indepth explanation! I’m glad there are people like you out there exposing the truth. We need more knowledgeable, moral people like you!
{And you’ve got to believe
{And you’ve got to believe the AG would have a major problem defending his release of GNC’s products for sale. That would stand as proof in most courts that he knew he was wrong to issue the cease and desist in the first place, that all of the products sold by the Gang of Four were what they said they were, and yet he still restricted the trade of three major corporations–purely for political reasons}: I just carefully read this entire article, & what stands out MOST in my mind is the above paragraph. Considering the indisputable fact that this jerk let GNC off the hook after they agreed to sign his bogus agreement, yet he left the cease & desist order intact for the other 3 companies who refused to cooperate with his BS, then from a purely rational viewpoint, I don’t see how he could ever win this thing. (Since I do purchase & consume a lot of supplements, I really hope I’m right!)
This is an awesome motivating
This is an awesome motivating article. I am practically satisfied with your great work. You put truly extremely supportive data. Keep it up. Continue blogging. Hoping to perusing your next post